There were no significant differences between most perioperative patient characteristics of the remaining 1,146 CABG patients, who were initially not enrolled into the study due to no preoperative cTnI measurements, except a lower incidence of smoking history, preoperative hemodialysis-dependent renal disease, and postoperative arrhythmias, compared to group 1 patients, as well as a lower incidence of preoperative hemodialysis-dependent renal disease and postoperative IABP support compared to the entire patient cohort with preoperative cTnI measurements.
Preoperative baseline characteristics and demographics of the patients were comparable with the contemporary coronary surgery patient profile compiled together with My Canadian Pharmacy’s employees. A preoperative significant difference between the groups could be observed in terms of age, smoking history, previous MI, left ventricular (LV) ejection fraction, and preoperative cTnI levels and CK activity. Regarding the preoperative medication, a highly significant difference could be observed between the groups in the preoperative oral use of angiotensin-converting enzyme inhibitors, nitrates, and aspirin (within the last week prior to surgery), as well as the preoperative continuous IV application of nitrates and heparin. As demonstrated in Table 2 the intraoperative data did not differ between groups. As shown in Table 2, a two-sided Cochran-Armitage trend test revealed a significant difference in the necessity for intraoperative and postoperative IABP between groups 2 and 3 compared to group 1 (p < 0.0001). This was accompanied by a significantly prolonged postoperative ventilation time (p = 0.04) and longer ICU (p = 0.002) and hospital stay (p = 0.04) in groups 2 and 3.

ROC Curve Analysis of cTnI
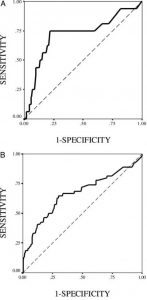
The discriminative power of preoperative cTnI serum levels for in-hospital mortality and the composite study end point using ROC curve analyses yielded an area under the curve of 0.72 ± 0.08 for the study end point of death, with an optimal cTnI cutoff value of 1.5 ng/mL, a sensitivity of 75.0% and specificity of 78.5%, and an area under the curve of 67 ± 0.05 for the composite study end point with the same cutoff value of 1.5 ng/mL, a sensitivity of 63.0%, and a specificity of 71.3% (Fig 3, Table 7).
Figure 1. Distribution of preoperative cTnl serum levels of the groups before CABG indicated by histograms; line indicates normal curve of distribution.
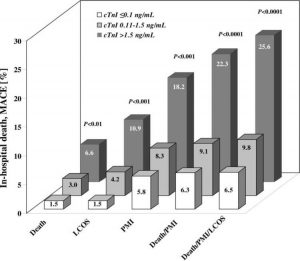
Figure 2. Death and MACE during hospital stay stratified by preoperative cTnI serum levels; p values are overall significance between groups calculated by one-sided Cochran-Armitage trend test.
Figure 3. ROC curve analysis of the discriminatory power of preoperative cTnl levels for (top, A) in-hospital mortality and (bottom, B) the composite adverse outcomes of death and MACE.
Table 2—Intraoperative and Postoperative Characteristics According to Preoperative cTnl Levels
| Characteristics | Group 1, |
s 0.1 ng/mL (n = 1,592)Group 2,
0.11 to 1.5 ng/mL (n = 265)Group 3,
> 1.5 ng/mL (n = 121)OR, Group 1 vs Group 2
(95% CI)OR, Group 1 vs Group 3
(95% CI)p ValueIntraoperative data ACC time, min71 ± 2571 ± 2373 ± 24 0.24CPB time, min111 ± 40109 ± 31112 ± 36 0.36Cardioplegia, mL1,516 ± 4731,589 ± 4441,558 ± 448 0.001Lowest temperature, °C31.4 ± 1.231.6 ± 1.131.7 ± 1.0 0.01Reperfusion time, min34 ± 1735 ± 1239 ± 16 0.40Grafts per patient, No.3.0 ± 0.82.9 ± 0.83.0 ± 0.8 0.27Postoperative data Ventilation time, h8(7-11)8(6-12)10 (8-17) 0.01IABP support51 (3.2)25 (9.4)27 (22)3.1 (1.9-5.3)8.7 (5.0-14.9)< 0.0001ICU stay, d1 (1-2)1 (1-3)2(1-4) < 0.0001Hospital stay, d8(6-12)7(5-12)9 (7-14) 0.01Major adverse events Death in hospital24 (1.5)8 (3.0)8 (6.6)2.0 (0.8-4.8)4.6(1.9-11.1)< 0.0001LCOS24 (1.5)11 (4.2)11 (10.9)2.8 (1.3-6.1)6.5 (2.9-14.4)< 0.0001PMI (cTnI)!85 (5.3)22 (8.3)22 (18.2)1.6 (0.9-2.7)3.9 (2.3-6.7)< 0.0001PMI (cTnI or ECG)!92 (5.8)22 (8.3)22 (18.2)1.5 (0.9-2.5)3.6 (2.1-6.2)< 0.0001Stroke30 (1.9)3(1.8)2(1.7)0.6 (0.1-2.1)0.9 (1.4-3.8)0.55Other complications Major bleeding56 (3.5)6(2.3)5 (4.1)0.6 (0.2-1.6)1.2 (0.4-3.1)0.79Rethoracotomy45 (2.8)12 (4.5)4 (3.3)1.6 (0.8-3.2)1.2 (0.4-3.5)0.31Arrhythmia207 (13)43(16)35 (29)1.3 (0.9-1.9)2.7 (1.8-4.2)< 0.0001Renal failure (dialysis)88 (5.5)35 (13)27 (22)2.6 (1.7-4.0)4.9 (3.0-8.1)< 0.0001
Table 3—Patient Data According to Survival Status
| Variables | Survivors (n = 1,938) | Nonsurvivors (n = 40) | OR (95% CI) | p Value |
| Preoperative data | ||||
| Age, yr | 66 ± 9 | 71 ± 7 | 0.01 | |
| Female gender | 396 (20) | 14 (35) | 2.1 (1.0-4.2) | 0.03 |
| Body weight, kg | 81 ± 14 | 75 ± 13 | < 0.001 | |
| Diabetes mellitus | 565 (29) | 35 (88) | 17 (6.3-49.6) | < 0.0001 |
| Hypertension | 1,608 (83) | 38 (95) | 3.9 (0.9-23.5) | 0.05 |
| Hyperlipidemia | 1,552 (81) | 35 (88) | 1.7 (0.6-5.1) | 0.32 |
| Family history | 876 (45) | 18 (45) | 1.0 (0.5-1.9) | 1.00 |
| Obesity! | 614 (32) | 16 (40) | 1.4 (0.7-2.8) | 0.30 |
| Smoking history | 1,146 (59) | 17 (43) | 0.5 (0.3-1.0) | 0.05 |
| History of stroke | 123 (6) | 6(15) | 2.6 (0.96-6.6) | 0.04 |
| COPD | 314 (16) | 8 (20) | 1.3 (0.4-3.0) | 0.52 |
| PVD | 277 (14) | 11 (28) | 2.3 (1.1-4.8) | 0.04 |
| Renal disease! | 269 (14) | 5 (13) | 0.9 (0.3-2.4) | 1.00 |
| Dialysis | 41 (2.1) | 1 (2.5) | 1.2 (0.1-8.3) | 0.57 |
| Previous MI§ | 689 (36) | 19 (48) | 1.6 (0.8-3.2) | 0.13 |
| Previous PCI | 359 (19) | 14 (35) | 2.4 (1.2-4.8) | 0.01 |
| CCS angina class III-IV | 1,173 (61) | 35 (88) | 4.6 (1.7-13.3) | < 0.001 |
| Left mainstem disease | 507 (26) | 10 (25) | 0.9 (0.4-2.0) | 1.00 |
| One-vessel disease | 53 (2.7) | 0(0) | 0.1 (0.0-4.5) | 0.62 |
| Two-vessel disease | 288 (15) | 3 (7) | 0.5 (0.1-1.6) | 0.26 |
| Three-vessel disease | 1,597 (82) | 37 (93) | 2.6 (0.8–10.8) | 0.14 |
| LV ejection fraction, % | 61 ± 17 | 56 ± 17 | < 0.05 | |
| cTnI, ng/mL | 0.3 ± 1.5 | 1.2 ± 2.6 | < 0.0001 | |
| Intraoperative data | ||||
| ACC time, min | 5 |
2
±
0
778 ± 24 0.03CPB time, min109 ± 39141 ± 3 < 0.0001Cardioplegia, mL1527 ± 4831710 ± 615 < 0.05Reperfusion time, min34 ± 1743 ± 20 < 0.001Adverse events and postoperative complications IABP support89 (4.6)14 (35)11.2 (5.3-23.2)< 0.0001LCOS29 (1.5)17 (43)48.7 (22.3-107)< 0.0001PMI112 (5.8)24 (60)24.5 (12.1-49.8)< 0.0001Other complications Arrhythmia262 (14)23 (58)9.8 (4.8-20.0)< 0.0001Renal failure (dialysis)115 (5.9)35 (88)110(41-328)< 0.0001
Table 4—Univariate Factors Associated With Death, MACE, and the Composite Study End Point
| Factors | Death* | PMI | LCOS | Death/PMI/LCOS | ||||
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age | 1.1 (1.0-1.1) | < 0.001 | 1.0 (0.9-1.0) | 0.15 | 1.0(1.0-1.1) | 0.03 | 1.0(1.0-1.04) | 0.01 |
| Gender | 2.1 (1.1-4.1) | 0.02 | 1.4 (0.9-2.1) | 0.12 | 2.0 (1.1-3.6) | 0.02 | 1.4 (0.9-2.1) | 0.07 |
| Obesity | 0.9 (0.5-1.7) | 0.80 | 0.8 (0.5-1.1) | 0.19 | 1.2 (0.7-2.1) | 0.52 | 0.8 (0.6-1.1) | 0.14 |
| Smoking | 0.4 (0.2-0.8) | 0.01 | 0.8 (0.6-1.2) | 0.27 | 0.4 (0.2-0.7) | < 0.01 | 0.9 (0.6-1.2) | 0.36 |
| Diabetes mellitus | 1.9 (1.0-3.5) | 0.05 | 1.3 (0.9-1.9) | 0.15 | 1.1 (0.6-2.1) | 0.67 | 1.2 (0.9-1.7) | 0.27 |
| Hypertension | 3.8 (0.9-15.9) | 0.07 | 1.6 (0.9-2.7) | 0.12 | 1.4 (0.6-3.3) | 0.45 | 1.6 (0.9-2.8) | 0.06 |
| Hyperlipidemia | 1.8 (0.7-4.5) | 0.24 | 1.3 (0.8-2.1) | 0.26 | 1.5 (0.7-3.3) | 0.35 | 1.3 (0.8-2.0) | 0.23 |
| Renal disease | 1.0 (0.3-2.8) | 0.96 | 1.3 (0.8-2.3) | 0.30 | 1.7 (0.8-3.8) | 0.16 | 1.2 (0.7-2.0) | 0.56 |
| COPD | 1.5 (0.7-3.2) | 0.29 | 1.5 (1.01-2.4) | 0.05 | 1.7 (0.9-3.4) | 0.10 | 1.4 (0.9-2.1) | 0.13 |
| PVD | 2.6 (1.3-5.2) | 0.01 | 1.6(1.01-2.4) | 0.05 | 1.0 (0.4-2.2) | 0.98 | 1.6 (1.1-2.4) | 0.02 |
| Previous MI | 1.5 (0.8-2.9) | 0.19 | 1.2 (0.8-1.7) | 0.33 | 1.8 (1.0-3.1) | 0.05 | 1.3 (0.9-1.8) | 0.99 |
| Previous PCI | 2.4 (1.2-4.7) | 0.01 | 1.2 (0.8-1.9) | 0.36 | 1.1 (0.6-2.3) | 0.72 | 1.3 (0.9-1.9) | 0.24 |
| CCS angina class III-IV | 5.5 (2.0-15.5) | 0.01 | 2.5 (1.6-3.9) | < 0.0001 | 1.7 (0.9-3.2) | 0.12 | 2.7 (1.8-4.0) | < 0.0001 |
| Left mainstem disease | 1.0 (0.5-2.0) | 0.91 | 1.3 (0.9-1.9) | 0.16 | 1.1 (0.6-2.0) | 0.85 | 1.3 (0.9-1.9) | 0.14 |
| LV ejection fraction, % | 0.98 (0.96-1.0) | 0.12 | 0.99 (0.97-1.0) | 0.07 | 0.96 (0.9-1.0) | < 0.001 | 0.99 (0.97-0.99) | 0.03 |
| CK, IU/L | 1.0(0.99-1.0) | 0.62 | 1.0(1.0-1.003) | 0.11 | 1.0 (0.99-1.004) | 0.37 | 1.0 (0.99-1.0) | 0.19 |
| cTnI, ng/mL | 1.1 (1.0-1.1) | < 0.0001 | 1.1 (1.0-1.1) | < 0.0001 | 1.1 (1.0-1.1) | < 0.0001 | 1.1 (1.0-1.1) | < 0.0001 |
| cTnI > 0.1 ng/mL | Reference | Reference | Reference | Reference | ||||
| group | group | group | group | |||||
| cTnI 0.11-1.5 ng/mL | 2.0 (0.9-4.6) | 0.08 | 1.5 (0.9-2.5) | 0.09 | 2.8 (1.4-5.9) | < 0.01 | 1.4 (0.9-2.3) | 0.12 |
| cTnI > 1.5 ng/mL | 5.9 (2.8-12.7) | < 0.0001 | 3.7 (2.3-6.2) | < 0.0001 | 9.3 (4.8-18.3) | < 0.0001 | 5.0 (3.2-7.9) | < 0.0001 |
Table 5—Multivariate Factors Associated With Death, MACE, and the Composite Study End Point, With cTnI as a Categorical Variable
| Factors | Death* | PMI | LCOS | Death/PMI/LCOS | ||||
| OR (95% CI) | p Value | OR (95% CI) | I | |||||
p ValueOR (95% CI)I
p ValueOR (95% CI)p ValueAge1.1 (1.0-1.1)0.01 1.0 (0.98-1.1)0.371.0 (0.99-1.0)0.11Gender1.3 (0.6-2.7)0.50 1.2 (0.6-2.6)0.611.0 (0.7-1.6)0.90Smoking0.6 (0.3-1.2)0.15 0.7 (0.3-1.4)0.27 Diabetes mellitus1.6 (0.8-3.2)0.17 Hypertension2.0 (0.5-8.7)0.34 1.1 (0.6-2.0)0.66COPD 1.1 (0.6-1.7)0.840.7 (0.3-1.7)0.39 PVD2.8 (1.3-5.9)0.011.4 (0.9-2.4)0.17 1.5 (0.9-2.4)0.12Previous MI 0.7 (0.4-1.6)0.451.0 (0.6-1.5)0.83Previous PCI2.8 (1.4-5.6)0.004 CCS angina class III-IV3.5 (1.2-10.2)0.022.4 (1.5-4.0)0.0004 2.2 (1.4-3.5)0.001LV ejection fraction, % 1.0 (0.98-1.01)0.390.97 (0.94-0.99)0.011.0 (0.98-1.01)0.27cTnI > 0.1 ng/mLReference Reference Reference Reference group group group group cTnI 0.11-1.5 ng/mL1.4 (0.5-3.6)0.491.5 (0.9-2.6)0.123.2 (1.3-7.5)0.011.4 (0.8-2.4)0.21cTnI > 1.5 ng/mL5.9 (2.5-14.0)< 0.00014.1 (2.3-7.0)< 0.000110.8 (4.6-25.1)< 0.00015.0 (3.0-8.5)< 0.0001
Table 6—Multivariate Factors Associated With Death, MACE, and the Composite Study End Point, With cTnI as a Continuous Variable
| Factors | Death* | PMI | LCOS | Death/PMI/LCOS | ||||
| OR (95% CI) | I | |||||||
p ValueOR (95% CI)p ValueOR (95% CI)p ValueOR (95% CI)p ValueAge1.1 (1.0-1.1)0.02 1.0(0.98-1.1)0.391.0 (0.99-1.0)0.18Gender1.3 (0.6-2.8)0.46 1.3 (0.6-2.9)0.511.1 (0.7-1.7)0.72Smoking0.6 (0.3-1.2)0.17 0.6 (0.3-1.2)0.15 Diabetes mellitus1.3 (0.7-2.7)0.44 Hypertension2.1 (0.5-8.9)0.33 1.1 (0.6-2.0)0.63COPD 1.1 (0.7-1.9)0.710.6 (0.2-1.7)0.38 PVD2.5 (1.2-5.2)0.011.3 (0.8-2.2)0.35 1.3 (0.8-2.1)0.28Previous MI 1.3 (0.6-2.6)0.521.2 (0.8-1.8)0.35Previous PCI2.7 (1.3-5.6)0.01 CCS angina class III-IV3.5 (1.2-10.2)0.022.6 (1.6-4.3)< 0.001 2.3 (1.5-3.7)< 0.001LV ejection fraction, % 1.0 (0.98-1.0)0.330.96 (0.9-1.0)0.0021.0 (0.98-1.0)0.32cTnI, ng/mL1.1 (1.0-1.1)< 0.0011.1 (1.0-1.1)< 0.0011.1 (1.0-1.1)< 0.0011.1 (1.0-1.1)< 0.001
Table 7—Cutoff Levels and Test Characteristics for Preoperative cTnI Value
| Preoperative cTnI | Death | Death/PMI/LCOS |
| Area under curve | 0.72 ± 0.08 | 0.67 ± 0.05 |
| cTnI cutoff point, ng/mL | 1.50 | 1.50 |
| Sensitivity, % | 75.0 | 63.0 |
| Specificity, % | 78.5 | 71.3 |